Abstract
Background. MicroRNAs (miRNAs) are significant regulators of human hematopoietic stem cells (HSC), and their deregulation contributes to hematological malignancies. Systematic analysis of miRNA expression and function during hematopoiesis has revealed regulatory loops where miRNAs contribute to hematopoietic differentiation and function. Among the identified miRNAs, miR-155 can regulate myelopoiesis and erythropoiesis and its overexpression along with the overexpression of miR-125 and miR-181 has been suggested to have functional effects in the regulation of HSC homeostasis.
Myelodysplastic syndromes (MDS) are characterized by dysfunctional HSC, ineffective blood cell production, progressive marrow failure, and exhibit a significant risk of progression to acute myeloid leukemia (AML). Although the pathogenesis of MDS has not been fully understood, various alterations of microRNAs (miRNAs) have been reported in MDS. There is recent evidence that the miRNAs mediated post-transcriptional control of gene expression is key component in the pathogenesis of MDS and AML. Several studies have reported that differentially expressed microRNAs are associated with the transformation of MDS to AML and clinical outcomes.
Aim. In this study we examined the potential prognostic impact of six previously described miRNAS (miR-181a-2-3p, miR-124-3p, miR-550a-3p, miR-155-5p, miR-151a-3p, and miR-125b-5p) on the pathogenesis of MDS.
Methods. Total RNA was extracted from diagnostic bone marrow samples of 41 MDS patients. Patients were stratified per the revised International Prognostic Scoring System (IPSS-R). After polyadenylation of 1 μg total RNA by poly(A) polymerase and subsequent reverse transcription with an oligo-dT adapter primer, miRNAs transcript levels were determined using a reverse transcription quantitative real-time PCR method, based on SYBR Green chemistry. U6 was used as reference gene. Relative microRNA transcript levels were calculated by the 2-ΔΔct method. The IBM SPSS statistics, version 26 (IBM Corporation, North Castle, NY, USA) was used for the statistical analysis of the results.
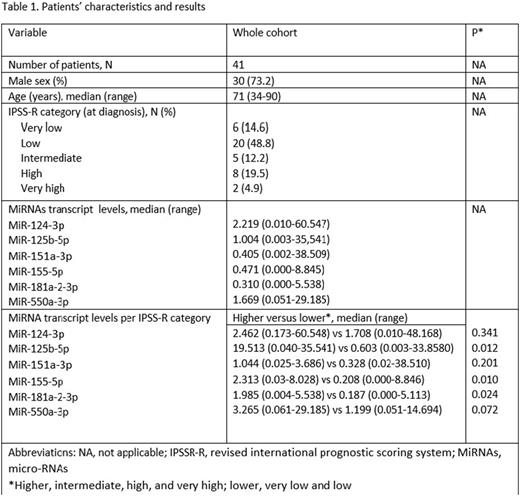
Results. The basic characteristics of the patients are shown in Table 1. Of the microRNAs analyzed in this study, miR-125b-5p, miR-155-5p, miR-181a-2-3p transcript levels were significantly elevated in higher (intermediate, high, and very high) risk MDS than in lower (very low and low) risk MDS (p=0.012, p=0.010, and p=0.024 respectively). MiR-125b-5p, miR-151a-3p, miR-155-5p, miR-181a-2-3p, and miR-550a-3p presented a positive correlation with the percentage of bone marrow blasts [p(0.001-0.05)], while a negative correlation was observed between the expression levels of mir-151-a-3p, miR181a-2-3p, miR-550a-3p, and the neutrophil count (p=0.05, 0.013, 0.010, and 0.007 respectively). None of the remaining factors that were tested (MDS type per the 2016 WHO classification, gender, age at diagnosis, hemoglobin levels, platelet count, and cytopenias) were correlated with the miRNAs levels.
Discussion. In this study we demonstrated the notable difference in the pattern of miRNA expression between lower and higher risk MDS patients. It has been shown that deregulation of miR-155-5p and miR-181a-2-3p may promote aberrant hematopoietic stem cell proliferation, differentiation, and progression to AML. Moreover, mice transplanted with miR-155-5p transfected stem cells developed a myeloproliferative disorder with abnormal granulocyte morphology analogous to MDS, suggesting a role in the higher risk MDS phenotype. Finally, it has been reported that miR-181a-3p was strongly expressed in the cytoplasm of high risk MDS cells, in contrast to low risk samples which displayed reduced levels. The identification of miRNAs that distinguish lower risk from higher risk MDS indicates that microRNA expression profile may be exploited to assist with making treatment decisions. Given that certain miRNAs can discriminate MDS subtypes and risk groups, it is not unlikely to think that an miRNA signature could be a useful tool for MDS risk stratification. Based on our current findings and other previously reported results, we continue the study in a larger cohort of patients. Targeting miRNAs may provide new treatment options for MDS
Disclosures
Diamantopoulos:Novartis, Roche, Janssen, BMS: Honoraria. Galanopoulos:abbvie,novartis: Consultancy, Honoraria. Ioannis:abbvie,novartis, bms: Honoraria, Research Funding. Symeonidis:Demo/Apopharma: Research Funding; Rafarm: Honoraria; Servier SOBI: Membership on an entity's Board of Directors or advisory committees; Astellas: Research Funding; Abbvie, Amgen, Astra-Zeneca, BMS, GenesisPharma, Gilead, Glaxo, Integris, Janssen, Novartis, Pfizer, Sanofi, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; WinMedica: Research Funding; Vianex: Research Funding; Roche: Research Funding. Panayiotidis:ABBVIE, NOVARTIS, GENESISPHARMA, BMS,Gilead, Glaxo, Integris, Janssen, Novartis, Pfizer: Honoraria, Research Funding. Viniou:ABBVIE, NOVARTIS, BMS,ASTRAZENECA, ASTELLAS GENESISPHARMA,: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal